10.03.2025
Why should you hammer nails into a lemon?
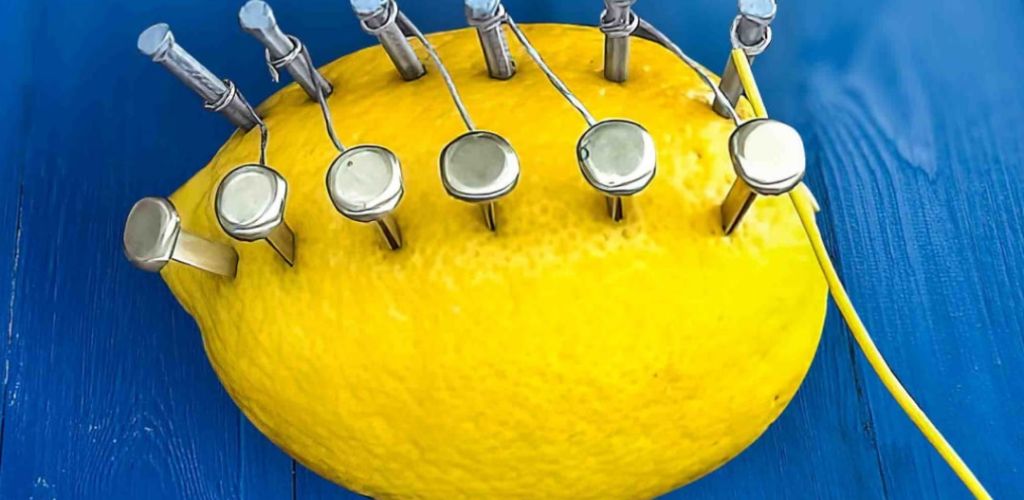
The reason you might hammer nails into a lemon is typically for a science experiment demonstrating how to create a simple battery. Here’s the science behind it:
The Lemon Battery:
- Chemical Reaction:
- The lemon acts as an electrolyte, a substance that conducts electricity.
- When you insert two different metals, like a galvanized nail (zinc) and a copper nail or penny, into the lemon, a chemical reaction occurs.
- The citric acid in the lemon reacts with the metals, causing electrons to flow.
- Creating a Circuit:
- This flow of electrons creates an electrical current.
- By connecting wires to the nails, you can complete a circuit and power a small device, like an LED light.
- Why Nails?:
- Galvanized nails provide the zinc needed for the chemical reaction.
- Copper nails or pennies provide the copper.
- In essence:
- It is not the lemon that makes the electricity. The electricity is produced by the chemical reaction between the metals and the acid in the lemon. The lemon is the electrolyte which enables the transfer of electrons.
Key Takeaways:
- This is a popular science experiment that demonstrates the principles of a voltaic cell (battery).
- It’s a great way to visually understand how chemical reactions can produce electricity.
Therefore the hammer is used to drive the metal objects into the fruit in order to create the conditions required for a small electrical charge to occur.